Background: Tazemetostat (TAZ), an enhancer of zeste homolog 2 (EZH2) inhibitor, is approved by the US Food and Drug Administration for the treatment of patients with relapsed/refractory (R/R) follicular lymphoma (FL) whose disease has mutant (MT) EZH2 and who have received ≥2 prior therapies or with R/R FL who have no satisfactory alternative treatment options. We previously reported that the RP2D of TAZ in combination with R-CHOP was 800mg BID in the phase 1 part of Epi-RCHOP study (NCT02889523). Here, we report results of the phase 2 part evaluating TAZ with R-CHOP + rituximab maintenance in a cohort of previously untreated high-risk FL patients.
Methods: patients were eligible if aged 18-80 years, had histologically confirmed FL grade 1-3A, high-risk FLIPI (score of 3-5) and were in need of treatment according to GELF criteria.
Induction treatment consisted in 6 cycles of RCHOP every 21 days and TAZ 800mg BID every day, starting on day 2 of cycle 1, plus 2 additional 21 days cycles of rituximab+TAZ (cycle 7 and 8). Responses were assessed at end of induction (EOI), and responders then received maintenance 1 (TAZ 6 mo + rituximab 12 mo) and maintenance 2 (12 mo of rituximab alone).The primary efficacy endpoint was PET-based complete response rate based on local assessment at the end of induction or at permanent treatment discontinuation, as determined by Cheson IWG 2014 Lugano Classification (Deauville scale 1-3); in addition bone marrow biopsy (BMB) was mandatory at EOI if involved at baseline and complete metabolic response (CMR) patients with missing assessment at EOI classified as partial responders. Sample size calculation was made with the expectation of an increase of 13% in CR, with an H0 hypothesis of 67% and an H1 assumption of 80%, leading to a theorical sample size of 62 pts, assuming a drop out of 10%. Initial assumption was that CR rate should be >76.4% to consider the combination eligible for further clinical evaluation in high risk FL patients.
Results: As of June 14, 2022, 62 patients were enrolled between Aug2020 and Apr2022 across 20 centers (including a drop-out of 12 patients, 70 were enrolled in France, 4 in Belgium). Disease characteristics included: males 61.3%, median age 64.5 years, FLIPI 3/4/5 in 61.3%/32.3%/6.5%, EZH2 mutated 17.3% (n=9/46 assessed had EZH2 mutation; 16 undertermined), BM involvement 68.3%, circulating FL cells in 21%, and increased LDH in 50%.
Fifty eight (93%) patients completed induction and initiated maintenance 1, 44 (71%) initiated maintenance 2. Fourteen patients permanently discontinued treatment: 4 before EOI not related to PD (2 COVID19 disease, 1 late onset neutropenia and 1 stopped therapy but accepted FU), and 9 after EOI (5 PD, 2 COVID19 disease, 1 neutropenia, 1 paralytic ileus and 1 patient's refusal).
At EOI, 79% (95%CI 66.8; 88.3%) achieved complete metabolic response (CMR) and 16.2% PMR for an overall metabolic response rate of 95.2%, however, according to the stringent protocol definition, 16 patients who achieved CMR had no BMB assessment at EOI and were downgraded to PR leading to 53.2% CR and 41.9% PR rate at EOI. Complete response rates were significantly higher in EZH2 mutants (8/9 CMR 88.9%) versus 17/37 (45.9%) in EZH2 wild-type (WT) patients (Fisher exact test p=0.027).
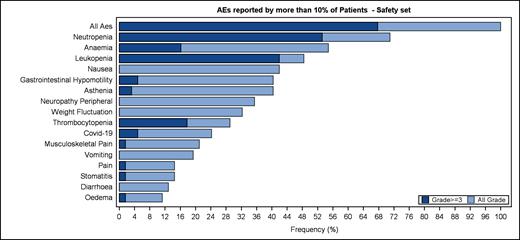
Figure 1 shows the AEs reported by more than 10% of patients. The most common grade 3-4 TEAEs were neutropenia (53.2%, only 3.2% febrile), thrombocytopenia (17.7%), anemia (16.1%). Serious AEs occurred in 14 patients, the two most frequent being Covid19 disease (6.5%) and gastrointestinal hypomotility (6.5%). This latter AE was the most frequent non-hematologic event (40.3%), followed by peripheral neuropathy (35.5%). After amending vincristine dose (Oct2020), 30 digestive AEs were reported (16 related to TAZ, mostly grade 1-2, only 1 grade 3, no sequelae). No SPM was observed.
With a median follow-up of 19 months, 7 relapses (5 EZH2 WT and 2 undetermined) including 5 needing subsequent treatment and 2 deaths occurred (1 Covid19 disease, 1 after PD). Median PFS and median DOR were not reached and appeared to be similar irrespective of EZH2 status, 18-months PFS and OS rates were 89.3% and 98.3% respectively.
Conclusions: Although the primary endpoint was not met due to missing BM assessments, TAZ + RCHOP combination demonstrated a promising complete metabolic response rate of 79% in this high-risk frontline FL population, with a manageable safety profile, after vincristine dose was reduced.
Disclosures
Ysebaert:Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Gilead/Kite: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Beigene: Honoraria, Research Funding, Speakers Bureau; Abbvie: Honoraria, Research Funding, Speakers Bureau. Brisou:Novartis: Consultancy. Bachy:Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Pfizer: Honoraria, Other: Personal Fees; Incyte: Honoraria; Takeda: Honoraria; Novartis: Honoraria, Other: Personal Fees; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees. Cartron:AbbVie: Consultancy, Honoraria; MedxCell, Ownards Therapeutics, MabQi, Emercell, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Consultancy; Jansen, Gilead, Novartis, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Honoraria; BMS: Consultancy, Honoraria; MabQi: Consultancy; MedxCell: Consultancy; Janssen: Honoraria; Gilead: Honoraria; Emercell: Consultancy; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Ownards Therapeutics: Consultancy; Roche: Consultancy, Honoraria. Carras:Janssen Cilag: Membership on an entity's Board of Directors or advisory committees, Other: travel fees, Research Funding; Kitegilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Other: Travelfees; Beigene: Membership on an entity's Board of Directors or advisory committees. Tessoulin:Incyte: Honoraria; Gilead: Honoraria; Abbvie: Honoraria; Kite: Honoraria. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Sarkozy:Beigene: Consultancy; Prelude Therapeutics: Consultancy; Roche: Other: Travel, Accommodations, Expenses, Research Funding; Gilead: Other: Congress fees; GSK: Consultancy; Incyte Bioscience: Consultancy, Other: Travel, Accommodations, Expenses; BMS: Consultancy; Janssen: Consultancy; AbbVie: Honoraria; Lilly: Honoraria; Gilead: Other: Travel, Accommodations, Expenses; Takeda: Other: Travel, Accommodations, Expenses. Laurent:Janssen Pharmaceuticals: Honoraria; F. Hoffmann-La Roche AG: Research Funding. Ribrag:AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Research Funding; Astex Pharmaceuticals: Research Funding; Argenx: Research Funding; Roche: Consultancy; NanoString: Consultancy; Incyte: Consultancy; Gilead: Consultancy. Morschhauser:Gilead: Consultancy, Other: Advisory Board; Janssen: Honoraria; AbbVie: Consultancy, Other: Advisory Board; BMS: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; Novartis: Consultancy, Other: Advisory Board; Incyte: Other: Advisory Board; Epizyme: Other: Advisory Board; Genmab: Consultancy, Other: Advisory Board; Roche: Consultancy, Honoraria, Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal